Lipid Self-Assemblies
Spontaneous Structural Diagrams of Bicellar Mixtures:
Bicellar mixtures are lipid mixtures composed of long- and short- chain lipids, which self-assemble into a variety of stru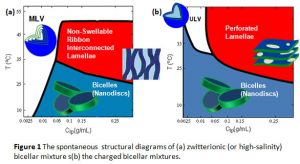 ctures including: nanodiscs (known as “bicelles”), unilamellar nano-vesicles, bilayered ribbons, alignable perforated lamellae, multilamellar vesicles, etc. Many features make “bicellar mixtures” attractive to the researchers in biochemical, biophysical and biomedical societies. First, the sizes in many of the nanoparticles are uniform at the nanoscale, e.g., well-defined radii of nanodiscs and nanovesicles, well-defined width of the bilayered ribbons. Moreover, the perforated lamellae of the bicellar mixtures under certain conditions are magnetically alignable, providing a way to align membrane-associated biomolecules in their native states (like a “goniometer”). Furthermore, the systems can serve ideal platforms for theragnostic carriers.
ctures including: nanodiscs (known as “bicelles”), unilamellar nano-vesicles, bilayered ribbons, alignable perforated lamellae, multilamellar vesicles, etc. Many features make “bicellar mixtures” attractive to the researchers in biochemical, biophysical and biomedical societies. First, the sizes in many of the nanoparticles are uniform at the nanoscale, e.g., well-defined radii of nanodiscs and nanovesicles, well-defined width of the bilayered ribbons. Moreover, the perforated lamellae of the bicellar mixtures under certain conditions are magnetically alignable, providing a way to align membrane-associated biomolecules in their native states (like a “goniometer”). Furthermore, the systems can serve ideal platforms for theragnostic carriers.
Some of the structures are kinetically trapped while others are possibly thermodynamically stable. Using small angle neutron scattering (SANS), we have successfully constructed spontaneous structural diagrams for a few mixtures as shown in Fig 1. A detailed explanation of some important morphologies is followed.
Fig. 2 illustrates a negatively-stained transmission electron microscopic (ns-TEM) image of uniform nanodiscs composed of long-chain (dimyristoyl phosphatidylcholine, DMPC) and short-chain (dihexanoyl phosphatidylcholine, DHPC) lipids (taken by Ming Li). The sketch indicates the proposed segregation of two lipids to form planar DMPC-rich bilayer (blue) and highly-curved DHPC-rich rim (green). The diameter and thickness of the nanodiscs are around 30 ~ 40 nm and 5 nm, respectively. The same discoidal structure has been observed in DMPC/Cholamidopropyl)dimethylammonio]-hydroxy-propanesulfonate (CHAPSO), dipalmitoyl phosphatidylcholine (DPPC)/DHPC systems under certain as well. The structure has been confirmed by small angle neutron scattering (SANS). This research is funded by NSF-CMMI nanomanufacturing (#1131587).
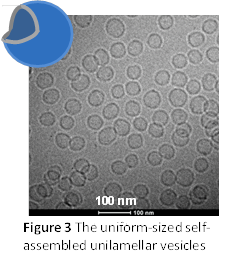
A nanodisc-to-nanovesicle transition takes place upon the elevation of temperature or dilution (see Fig 1). The vesicles normally inherit the uniform size from the nanodiscs and have radii in the range of 15 to 50 nm with a low polydispersity (standard deviation/average radius) < 30%. Fig 3 shows a cryogenic TEM (cryo-TEM) micrograph of a high-temperature charged DPPC/DHPC system, taken by Jaspreet Arora in Prof. Vijay John’s group at Tulane University. This structure has also been confirmed by SANS. It should be noted that the surface of the vesicles can be easily modified through hydrophobic anchoring of amphiphilic molecules or chemical conjugation.
Bilayered Ribbons:
Bilayer ribbons are normally found in non-charged lipid mixtures or high-salinity solutions around the melting transition temperature of the long-chain lipid. Fig 4 indicates a sketch of the bilayered ribbons, where long-chain lipids form the bilayer and short-chain lipids form the high-curvature edge. These ribbons can inter-connect with each other forming meshed-lamellae (as shown in Fig 1), which are alignable in a moderate magnetic field with their bilayer normal perpendicular to the magnetic field. Our recent study also shows that ribbons are more stable in DMPC/CHAPO than in DMPC/DHPC systems.
Alignable Lamellae in Solutions:
Most of the lipid membranes in solution form multi-lamellar vesicles, which are not alignable in solutions, while the bicellar mixtures are magnetically alignable in solutions with their bilayer normal perpendicular to the magnetic field, facilitating the structural analysis of membrane-associated proteins in physiologically relevant conditions. Such alignment is switchable through doping the lipid mixture with a small amount of paramagnetic ions (e.g., lanthanide group), resulting in the bilayer normal parallel with the magnetic field. The lamellae are presumably perforated (swiss-cheese like) bilayers with the short-chain lipids stabilizing the pore (defect) edges as shown in Fig 5. Recently, we have also found that shear flow is also capable of aligning free-standing membranes in solutions of lipid mixtures, providing an easier approach to align lipid membranes.