Interaction Between NPs And Cancer Cells/Tumor Tissues
Scientific Significance: The structures of the aforementioned lipid NPs are well-controlled, thus allowing us to study the interaction between NPs and cancer cells/tumor tissues. We have also designed tumor-on-a-chip to further the knowledge of NP diffusion in tumor tissues.
Applications: Optimization of the NP morphology for therapeutic/diagnostic delivery
Theranostic Delivery Nanocarriers:
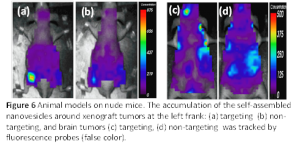
Both bicellar nanodiscs and nanovesicles can entrap lipophilic molecules (e.g., Nile Red, cholesterol…), while the latter can also encapsulate hydrophilic molecules in the interior water core. Therefore, they can be used as therapeutic/diagnostic delivery carriers. Fig. 6 demonstrates promising results of the in vivo studies using the self-assembled, antibody-conjugated nanovesicles containing both fluorescence dyes (Cy5.5) and MRI (magnetic resonance imaging) contrast agent. In all cases, the nanovesicles were injected through the tail vein of the mice. Both xenograft tumor [Figs 6 (a) and (b)] and brain tumor [Fig 6 (c) and (d)] models indicate significant accumulation of nanovesicles when their surfaces were conjugated with appropriate antibodies. It paves a potential pathway for NPs to go across blood brain barrier (BBB).
Although targeting nanovesicles show encouraging results, more effective cellular uptakes were found using nanodiscs in an in vitro study compared to nanovesicles. Fig 7(a) and (b) (collected by Wafa Aresh) show the fluorescence optical microscopic images of three different cancer cell lines: CCRF-CEM (top), KB (middle) or ovarian (bottom) to illustrate the cellular uptakes of bicellar nanodiscs and nanovesicles, respectively. Both NPs (namely nanodiscs and nanovesicles) had the identical chemical composition (Nile Red/lipids) and concentration, and were incubated with cancer cells at 37 oC for two hours. After being washed and centrifuged, the cells were monitored using optical microscopy under the same configuration with red (left), blue (middle) or combined (right) channel. Hoechst was also used to dye the nuclei of the cells. Evidently the cellular internalization of bicellar nanodiscs is much more than that of nanovesicles.
Tumors-on-A-Chip:
A microfluidic device containing tumors in hydrogel is developed in collaboration with Prof. Pinar Zorlutuna (at the Univ. Notre Dame) to mimic the physiological environment of tumors embedded in the extra-cellular matrixes (ECM) as shown in the video of Fig 8. The result also indicates that nanodiscs diffuse more effectively through the ECM than the nanovesicles do